Structural Biology
Proteins are responsible for all life phenomena. We study protein structures using cryo-electron microscopy and synchrotron radiation X-rays to understand protein function better. The knowledge gained will help us to understand protein functions profoundly and to create new catalysts and technologies.
|
|
 |
|
|
|
|
|
|
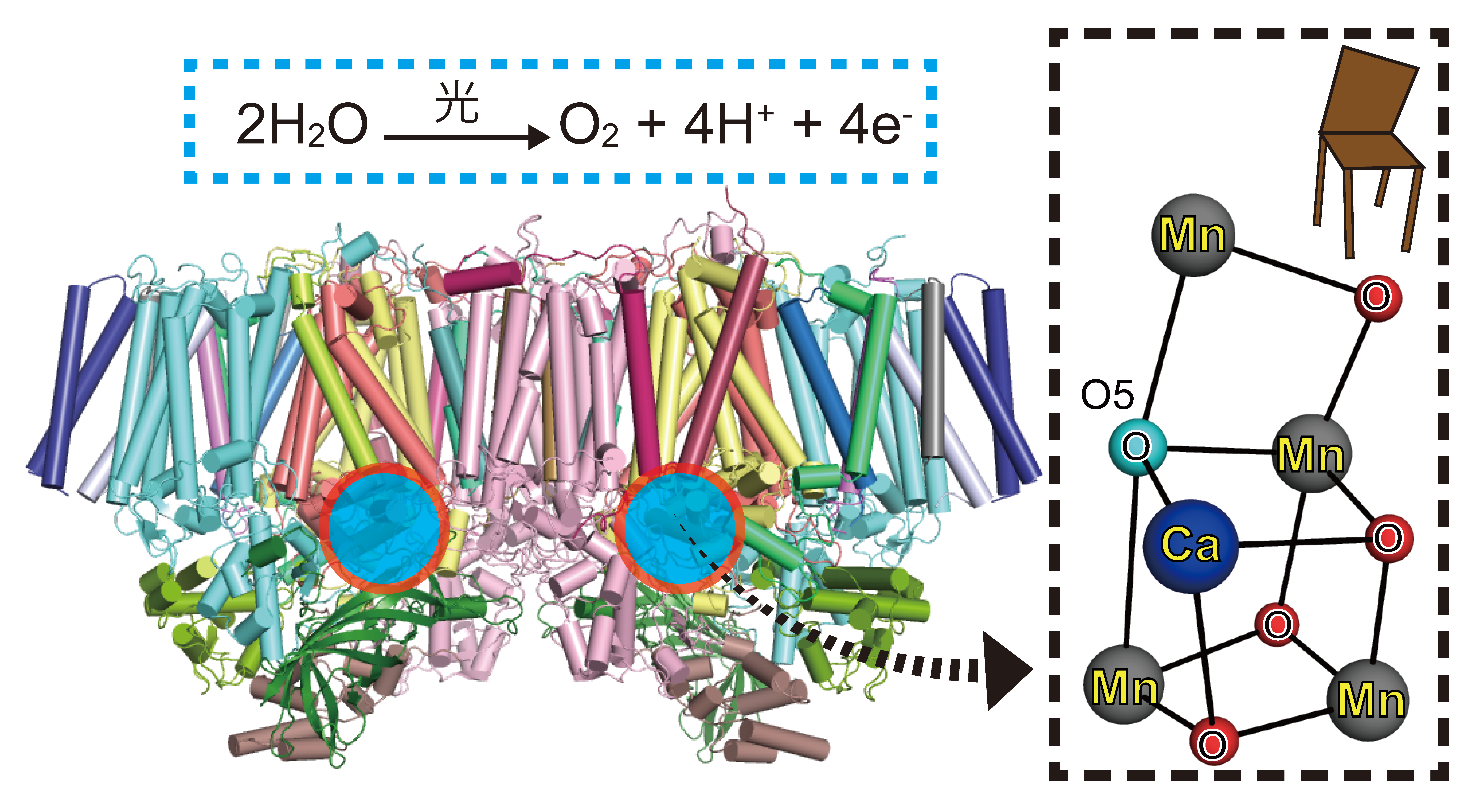
Photosynthesis is a reaction that converts light energy into chemical energy performed by plants and algae and supports the survival of almost all living organisms. In photosynthesis, huge protein complexes called photosystem II absorb the light energy and efficiently utilize it to extract electrons and hydrogen ions from water and release oxygen. Water molecules, which exist everywhere on earth, are stable, and oxidizing (splitting) them is not easy. The catalytic part of Photosystem II, called the manganese cluster, catalyzes the water-splitting reaction of Photosystem II. The manganese cluster proceeds through five cyclic intermediate states called the Si state cycle (i = 0-4), evolving oxygen molecules in the S3→S4→S0 states. We are elucidating the oxygen evolving reaction in Photosystem II using the cutting edge of X-ray crystallography and cryo-electron microscopy.