Theoretical Physical Chemistry
By using theoretical approaches such as statistical mechanics, thermodynamics, and molecular simulation, we are tackling a wide range of research issues related to liquids, solutions, interfaces, phase transitions, polymers, proteins, viruses, and cells. The latest research topics include solute size dependence of hydrophobic interactions, solubility of solutes, effective interactions, ion-specific effects on phase separation, structure of interfaces near the triple critical point of three-phase equilibrium systems, structural stability of proteins, co-solvent effects on proteins, and design principle of biological molecular motors and their efficiency of the free energy transduction, etc.
 |
|
|---|
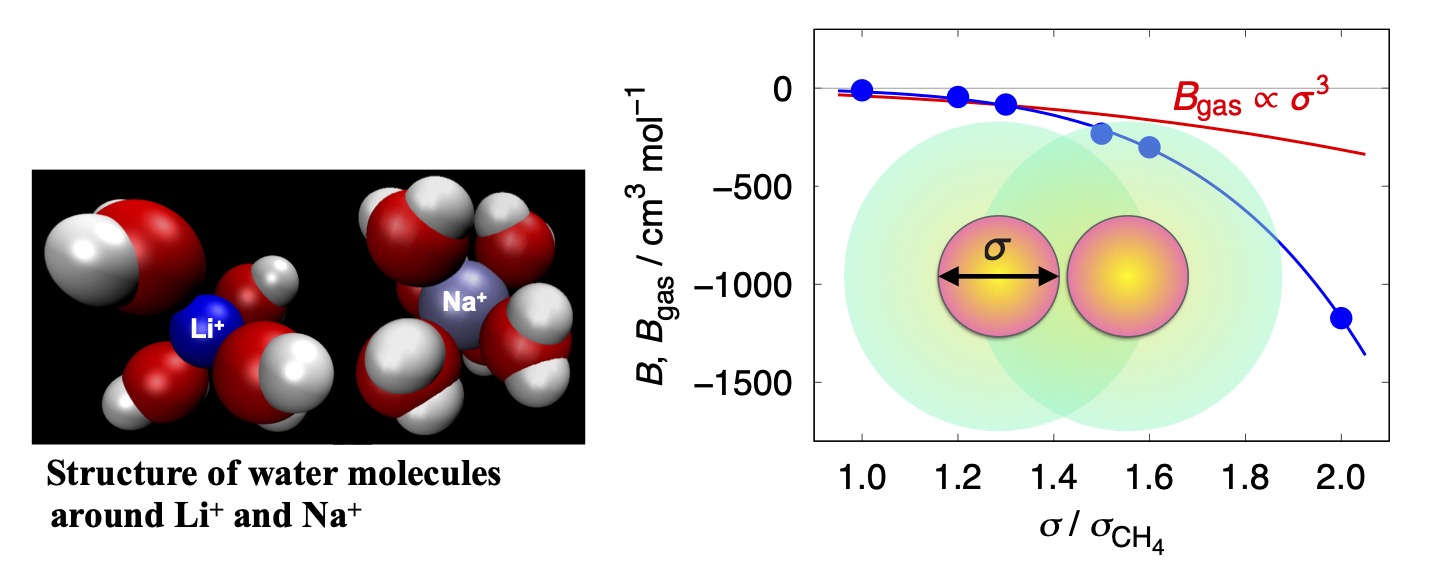
Theoretical studies on the ion-specific effect and the solute-size effect
The ion-specific effect is effects of ions on solubility of solutes, surface tension, stability of solutions that cannot be explained just by the valence or concentration of ions. The ion-specific effect plays important roles in sea water, soft mater, and water in physiological systems.We examined a simple question as to why LiCl has a weaker salting-out effect than NaCl, and we presented a microscopic mechanism. Another topic concerns a question: how do solvent-mediated interactions between solute particles, such as hydrophobic interactions and colloid-colloid interactions, change with the solute size? Calculating the osmotic second virial coefficient for particles in liquids, we try to find an analytical expression for the size dependence of the virial coefficient.