Functional Organic Chemistry
By using transition metal catalysts, we can develop the organometallic reagents and the organometallic complexes which show the reactivities and selectivities different than ones in classical methods. We can also control the reactivities of the reagents and the catalysts precisely by tuning the ligands (organic compounds) on the metals. We are pursuing the development of the new carbon-carbon bond formation reactions which become a basis for synthetic organic reactions by talking advantage of the characteristics of the organometallics complexes which consist of the metals and the organic compounds.
 |
|
|---|
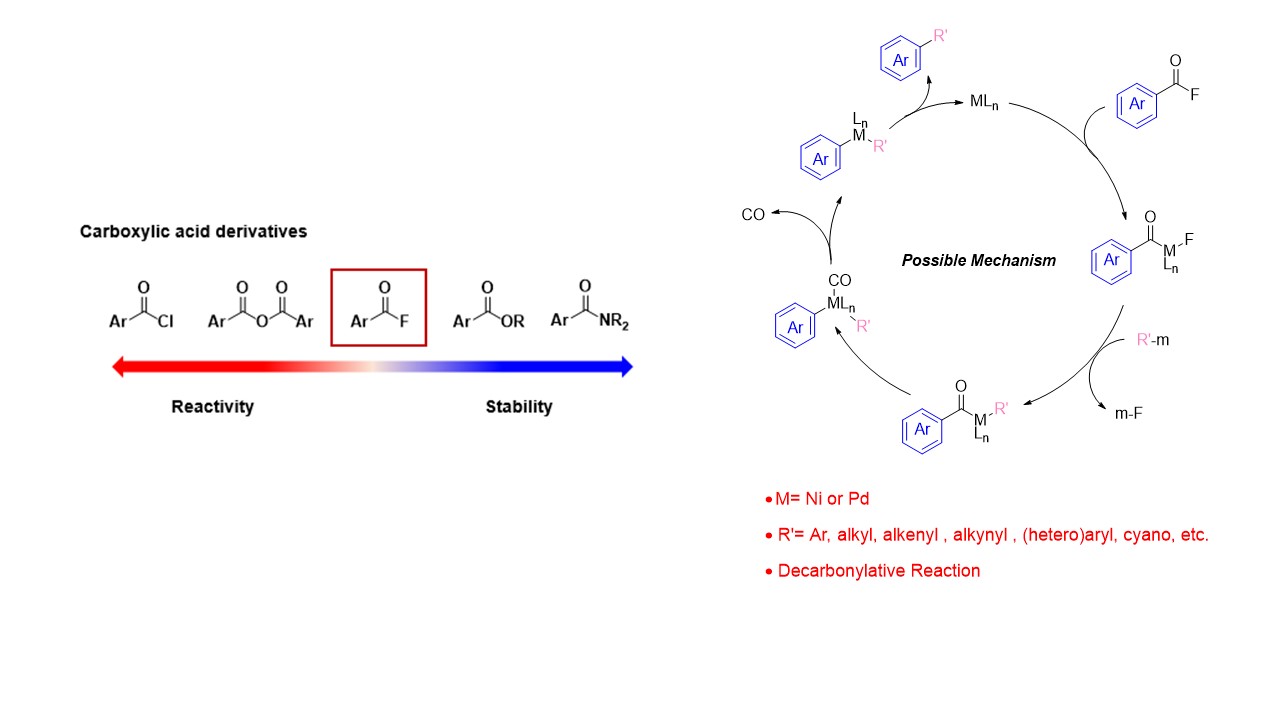
1. Nickel- or Palladium-catalyzed Decarbonylative Transformation of Acyl Fluorides (Nishihara)
Although acyl fluorides are easily prepared from the corresponding carboxylic acids, little research has been done on the reactivity of acyl fluorides so far. In order to develop transformation of acyl fluoride, we have succeeded the development of nickel- or palladium-catalyzed decarbonylative carbon-carbon and carbon-hetero atom bond formation reaction of acyl fluoride. These reactions may proceed through oxidative addition, transmetallation, and reductive elimination, and we utilize the isolation of intermediates and computational chemistry to clarify the stage at which the key decarbonylation reaction proceeds.
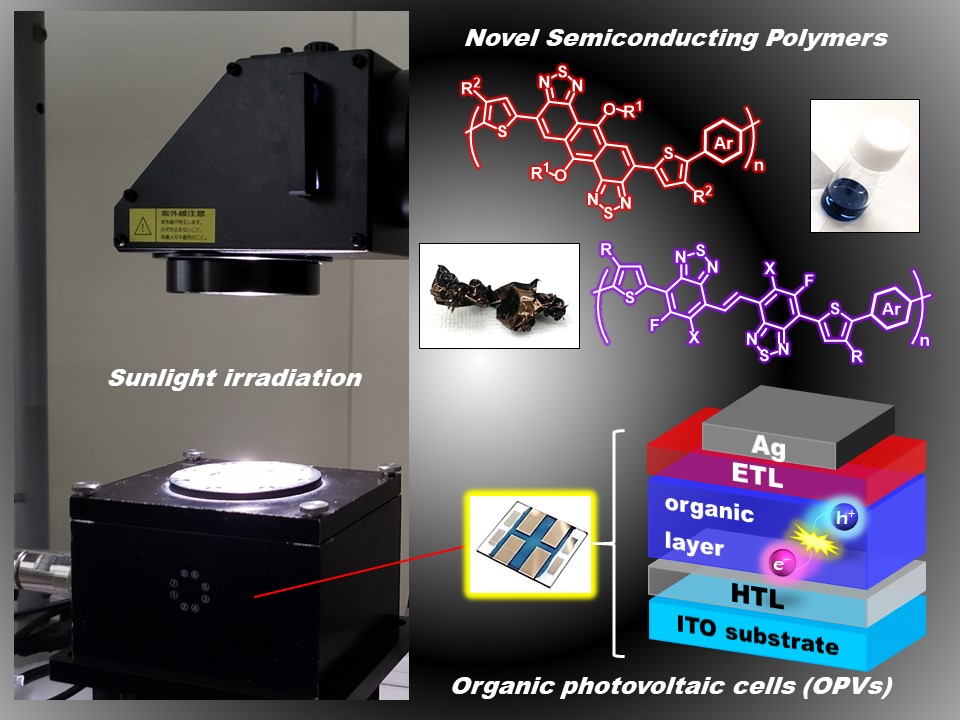
2. Development of semiconducting polymers based on novel electron-deficient heteropolycyclic aromatic frameworks (Mori)
Since heteropolycyclic aromatic compounds including thiadiazole rings have high electron-accepting nature, they are useful building blocks for high-performance p-type and n-type semiconducting polymers. We have successfully synthesized novel electron-deficient heteropolycyclic aromatic compounds containing thiadiazole rings, and their copolymers. The synthesized polymers exhibited good organic photovoltaic (OPV) properties.
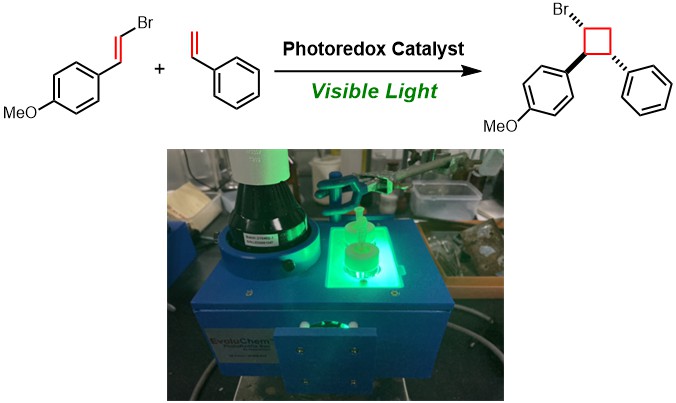
3. Visible-light-induced [2+2] Cycloaddition Reactions via Photoredox Catalysis (Tanaka)
[2 + 2] Cycloaddition reactions are one of the straightforward methods to provide cyclobutanes. While a classical synthetic tool for photoinduced [2 + 2] cycloadditions is the use of a high-energy light source such as ultraviolet light, we have successfully developed the visible-light-induced [2 + 2] cycloaddition of styrenes via photoredox catalysis. The photoredox catalyst selectively oxidized electron-rich styrenes to furnish polysubstituted cyclobutanes.
 |
|
|---|
2. Development of semiconducting polymers based on novel electron-deficient heteropolycyclic aromatic frameworks (Mori)
Since heteropolycyclic aromatic compounds including thiadiazole rings have high electron-accepting nature, they are useful building blocks for high-performance p-type and n-type semiconducting polymers. We have successfully synthesized novel electron-deficient heteropolycyclic aromatic compounds containing thiadiazole rings, and their copolymers. The synthesized polymers exhibited good organic photovoltaic (OPV) properties.
 |
|
|---|
3. Visible-light-induced [2+2] Cycloaddition Reactions via Photoredox Catalysis (Tanaka)
[2 + 2] Cycloaddition reactions are one of the straightforward methods to provide cyclobutanes. While a classical synthetic tool for photoinduced [2 + 2] cycloadditions is the use of a high-energy light source such as ultraviolet light, we have successfully developed the visible-light-induced [2 + 2] cycloaddition of styrenes via photoredox catalysis. The photoredox catalyst selectively oxidized electron-rich styrenes to furnish polysubstituted cyclobutanes.